Several mad scientists have struck our imagination with their science experiments that had catastrophic consequences. Think of Doctor Frankenstein, who gave life to a monster made from human remains, Doctor Octopus whose body was fused to mechanical arms, or Doc Brown, who accidentally made Marty McFly travel back in time!
In reality, the research carried out by scientists is (fortunately!) strictly overseen in Québec. Each research project must be approved by a Research Ethics Board (REB), which is itself supervised by the “Ministère de la santé et des services sociaux du Québec” or a postsecondary institution recognized by the “Ministère de l’Enseignement Supérieur du Québec.” The REB assesses potential ethical issues of the project, including the risks incurred by the participants, and will also ensure its scientific quality.
REBs are “responsible for evaluating the compliance of research projects with ethical rules, ensuring their ethical monitoring and ensuring the protection of individuals” (Translation, PAM, 1998). The REBs make sure that research projects adhere to three guiding principles: the respect for people, concern for well-being, and justice. They also aim to strike an appropriate balance between the protection of participants and the recognition of the potential benefits arising from the research (TCPS 2, 2018).
In order to ensure a quality and fair evaluation, the REBs are made up of at least 5 members: two members experienced in the research disciplines evaluated by the REB in question (the REBs have specific areas of expertise), one member specializing in ethics, one member specializing in law and a member of the community (TCPS 2, 2018).
The CERVO research center, in which our laboratory is located, is part of the CIUSSS de la Capitale-Nationale (CIUSSS-CN). The CIUSSS-CN has four REBs, related to the four research centers that it houses : sectoral REB for young people facing difficulties and their families, sectoral REB in rehabilitation and social integration, sectoral REB in population health and frontline and sectoral REB in Neuroscience and Mental Health. It is this latter REB that evaluates our laboratory’s research projects.
Here is an overview of the ethical approval process, including the preliminary steps to be carried out by the research teams:
Before the start of the project…
- The team writes up a research protocol.
The protocol lists each member of the research team, includes the rationalefor the study (i.e. justification/relevance) and its objectives, the hypotheses and methodology of the study, all details pertaining to how it will be carried out, as well as possible risks. For example, in the context of human research, such as in our laboratory, the protocol must contain the inclusion and exclusion criteria for the selection of participants, the number of visits that will be required, the location and duration of these visits, and a description of all tests and procedures that will be used. The inclusion and exclusion criteria, as well as the use of each test, must be justified. Following the principle of justice, it would not be acceptable to prevent people from participating in a research project without a valid scientific reason. Similarly, the people that participate in the studies should be a diverse group so that the weight of the research does not rest unduly on a group, as was the case in the past. The protocol also details all measures that will be taken to respect the confidentiality of the information that will be collected and to ensure the security of the project data. Finally, the protocol also details the analyses that are planned and the expected results.
- The team writes up an information and consent form intended for the participants.
This consent form must contain all the information that those who are interested in the study need in order to decide whether or not to participate. This information includes the names of the people responsible for the research project, the funding sources, the objectives and methodology of the study, the potential risks and disadvantages, the possible individual advantages (if any), how the data will be saved, protected and used as well as the participants’ rights. For example, in any consent form, it is stated that individuals participating in the study are free to withdraw from the research at any time. If the study provides a compensatory allowance (e.g. financial compensation for travel), it is also mentioned on this form. Compensation must be proportionate to the inconvenience. The REB ensures that compensation is adequate. The consent form must be written in plain language.
- The team provides the REB with all the documentation relating to the project.
In addition to the documents mentioned above, the team should provide the tools they wish to use to recruit participants (e.g. posters and emails, telephone interview questionnaires) and a copy of each questionnaire or test that participants will complete during the study. If the project has undergone a peer review – that is, an assessment of its scientific quality by other researchers – a copy of this assessment is sent to the REB.
- The REB evaluates the project and transmits the result of their evaluation to the research team.
The committee members evaluate the documentation provided, paying special attention to ethical issues. A full REB meeting is then held to discuss the projects. Researchers may be invited to join the committee meeting to provide more information about their project and to answer the committee’s questions. The REB may then approve, reject or require modifications to the research project based on its ethical acceptability. If necessary, the corrections must be made by the research team. Under no circumstance can the recruitment of participants begin until the ethics approval has been provided in writing by the committee. The Committee may request modifications several times and will only give its authorization when all the issues raised have been answered to its satisfaction.
- Following the REB’s evaluation, an “institutional evaluation” is carried out.
The purpose of this evaluation is to ensure that the people targeted by the research project and the patient treated at the institution’s match (if applicable), the availability of the establishment’s facilities, equipment and human resources that the project requires (if applicable), the financial aspects of the project and, if applicable, their impact on the institution’s budget, and the possibility of an alignment between the project and the institution’s orientations.
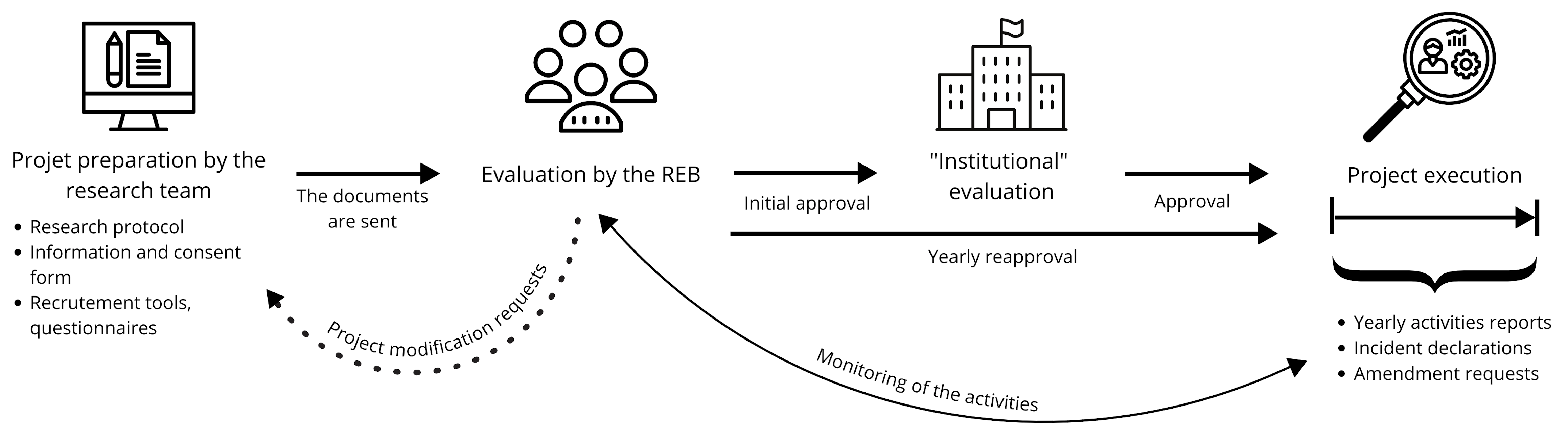
Figure 1. Diagram summarizing the steps of the steps of the ethics approval process.
During the project…
- The REB monitors the activities of the research project.
For projects spanning several years, the REB requires at a minimum, an annual report to be completed by the project manager (i.e. the researcher in charge of the project), conditional on obtaining the renewal of the ethical approval and thus, the continuation of the project. In the annual report, the research team indicates in particular the status of the project (e.g. recruitment in progress or completed), the number of participants recruited, provides a summary of research activities, etc. For projects that last less than a year, a final report is required at the end of the project (TCPS 2, 2018). - The researcher responsible for the project is obligated to report to the REB any significant modification to the research protocol and any incident likely to increase the level of risk the participants are exposed to.
To modify any component of the project, the team must complete an amendment form including justifications for the changes they wish to make. Formal approval must be obtained before any changes are made. In the case of an incident, the research team must inform the committee of the situation and its consequences (if any), and the measures taken following the incident. Depending on the nature of the incident, protocol changes may be required by the REB to minimize the risk of the incident recurring (TCPS 2, 2018). Temporary or permanent discontinuation of the project might also be required, if the level of risk for the participants become too high against the potential benefits of research (i.e. if the ratio risk–benefit ratio increases). For research projects for which there is no immediate benefit to the participant (class 4 studies), the level of risk that is acceptable is minimal to none. As potential benefit emerges (for example in clinical studies), the level of risk that is deemed acceptable increases (Biotech Health).
As you can see, the ethics approval process for research with human participants in Quebec is very rigorous and can take several months to complete. The initial evaluation as well as the follow-ups carried out by the REBs are mechanisms in place to protect research participants. In the lab, in addition to complying with all these measures, we also ensure that each team member is trained to detect potential ethical issues in our projects in order to avoid inappropriate behaviour, report problematic situations and to know how to react appropriately in such a situation.
References:
PAM: MSSS, Plan d’action ministériel en éthique de la recherche et en intégrité scientifique (PAM), (1998)
TCPS 2: Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans, december 2018 (Canadian Institutes of Health Research, Natural Sciences and Engineering Research Council of Canada, Social Sciences and Humanities Research Council)
Resources related to research ethics at the CIUSSS-CN (in French only):